WHAT IS CaHA FILLER?

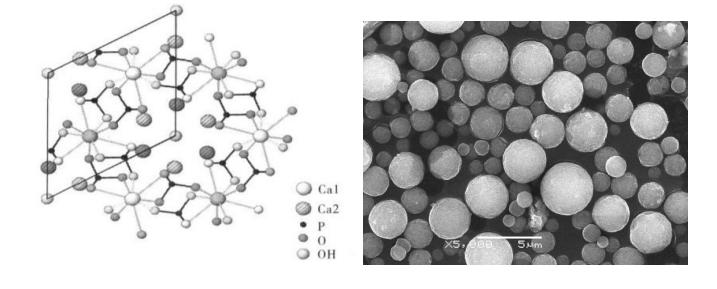
CaHA stands for Calcium Hydroxylapatite, which is a substance used in cosmetic procedures, particularly in dermal fillers. It is a biocompatible material that has been approved by regulatory bodies in various countries for use in filling deep lines and wrinkles, as well as for adding volume to certain areas of the face. When injected into the skin, CaHA stimulates collagen production, which can help improve skin texture and firmness over time. It's a popular option for those seeking non-surgical facial rejuvenation.
WHY CHOOSE CaHA FILLER?
Choosing a CaHA filler can be advantageous for several reasons:
Stimulates collagen production: CaHA stimulates the body's natural collagen production, leading to gradual improvements in skin texture and firmness over time. This can result in more natural-looking and longer-lasting results compared to fillers that simply add volume.

Biocompatibility: CaHA is a biocompatible material, with metabolic products (Ca²⁺, PO₄³⁻) that are naturally present in the body. This demonstrates its high biocompatibility, meaning it is well-tolerated by the body and carries a low risk of allergic reactions or other adverse effects.
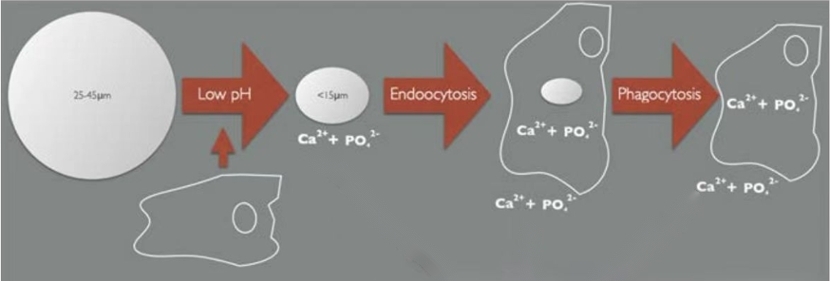
*In vivo metabolic pathways of CaHA
Gradual degradation: Over time, the CaHA particles in the filler are gradually broken down by the body and metabolized, leading to a natural-looking decline in the effects of the filler. Studies have shown CaHA could be degraded within human body 30 months post-injection, proving its excellent biodegradability.
Long-lasting results: Calcium hydroxylapatite fillers typically provide longer-lasting results compared to some other types of fillers. The effects can last up to a year or even longer in some cases.
WHAT'S THE DIFFERENCES OF CaHA FILLER AND TRADITIONAL HA FILLER?
The main differences between Calcium Hydroxylapatite (CaHA) fillers and traditional Hyaluronic Acid (HA) fillers lie in:
Longevity: CaHA fillers typically provide longer-lasting results compared to traditional HA fillers. The effects of CaHA fillers can last up to a year or more, whereas HA fillers usually last around 6 to 18 months, depending on the specific product and treatment area.
Mechanism of action: CaHA fillers work by providing immediate volume to the treated area while also stimulating the body's natural collagen production. This collagen stimulation helps improve skin texture and firmness over time. HA fillers primarily work by attracting and retaining water molecules, thereby adding volume to the treated area. They do not stimulate collagen production to the same extent as CaHA fillers.
Both types of fillers have their own advantages and are suitable for different individuals and treatment goals. The choice between CaHA and HA fillers depends on factors such as the specific concerns being addressed, desired duration of results, and individual preferences. It's essential to consult with a qualified healthcare provider to determine the most suitable filler for your needs.
WHAT IS HYAMAX® CaHA Lido?
HYAMAX® CaHA Lido is an innovative hybrid biostimulator and advanced injectable treatment that combines hyaluronic acid (HA) to enhance immediate volume increase with calcium hydroxyapatite (CaHA) for long-term collagen stimulation. This award-winning treatment visibly tightens and lifts the jawline and cheeks, delivering youthful and natural-looking results. The addition of Lidocaine in HYAMAX® CaHA Lido enhances the patient experience by reducing discomfort and pain during the procedure.